Olivier Elemento, Director of the Englander Institute for Precision Medicine
Background & Unmet Need
- Severe adverse events (SAEs) are the main reason drug development pipelines fail or drugs are withdrawn post approval
- Although these events should be detected in vitro, in vivo, or through clinical trials, they continue to arise unexpectedly at different stages of drug development, resulting in costly clinical trial failures and market withdrawal
- Dr. Olivier Elemento is actively developing an AI driven approach to better predict SAEs
- Unmet Need: Better methods for predicting clinical and pre-clinical safety and anticipate SAEs before they occur
- Dr. Elemento proposes to develop a data-driven machine learning approach that is derived from their highly cited PrOCTOR method1
- Their team plans to integrate information on a compound’s structure, target, and phenotypic effects with tissue-wide genomic profiling and their toxic target database to predict the probability of SAEs
- The program can also be used to screen all potentially targetable genes to identify toxic targets across multiple tissue types
- Ultimately, this work will enable the team to create a broadly applicable framework to not only predict toxic targets and SAEs but also accelerate the drug development pipeline and drive the design of new safer compounds
- Development Status: Dr. Elemento and his team have already established a proof of concept model and are expanding their program to integrate more data types and datasets
Project Goals
- The new framework will be tested on 6 different types of SAEs to assess accuracy, sensitivity, and specificity
- The method will be tested on approved but later recalled drugs to evaluate if they can predict SAEs missed by traditional approaches
- The team is planning to extend the program to better incorporate compound dosage and predict toxicities for drug and compound combinations in the coming months
Partnering Opportunity
Weill Cornell Medicine is seeking an industrial partner with expertise in drug development to advance the program and contribute data sets to further validate the approach
Supporting Data / Figures
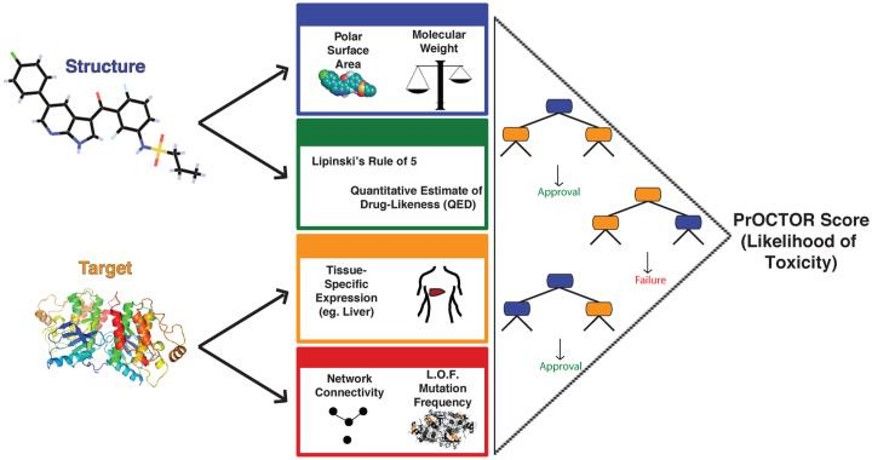
Figure 1: PrOCTOR Method Schematic. The PrOCTOR approach integrates chemical properties, drug-likeness measures and target-based properties of a molecule into a random forest model to predict whether the drug is likely to fail clinical trials for toxicity reasons.
Publications
Contact Information

For additional information please contact
James Bellush
Manager, Scientific Scouting
Phone: (646) 825-3681
Email: james.bellush@cornell.edu

