Vivek Mittal, Ford-Isom Research Professor of Cardiothoracic Surgery
Background & Unmet Need
- Triple Negative Breast Cancer (TNBC) is a particularly aggressive cancer with poor prognosis, which lacks effective broad-based therapies
- Over 90% of breast cancer deaths are due to metastases, which primarily occur due to resistance to chemotherapy and immunotherapy
- Copper is crucial in tumor progression and metastasis, and supports multiple TNBC resistance pathways in the tumor microenvironment, including:
- Lysyl oxidase-mediated stromal remodeling, which stiffens collagen and impedes T cell infiltration
- Enrichment of oxidative phosphorylation
- Enrichment of highly metastatic cells within primary tumors that initiate metastasis
- Unmet Need: New therapeutic strategies targeting resistance pathways for treatment of TNBC
Technology Overview
- The Technology: A therapeutic strategy for TNBC combining copper depletion via Tetrathiomolybdate (TM) with pembrolizumab and chemotherapy
- PoC Data: A 75-patient phase II pilot trial in late-stage cancer met its primary endpoints of decreased copper levels and reduction of VEGFR2+ endothelial progenitor cells, which initiate the ‘angiogenic switch’
- Among stage II/III and stage IV TNBC patients with no signs of disease after standard treatment, the event-free survival rates after an average follow-up of 6.3 years were 90% and 50%, respectively
- This far surpasses the average survival rate of 11% at 5 years for distant metastatic TNBC patients1
- Preclinical studies demonstrate that TM reverses key resistance pathways by decreasing collagen density, increasing immune cell infiltration, and shifting cells towards glycolysis
- A phase Ib/II clinical trial assessing this combination strategy in patients with residual TNBC is planned
Technology Applications
- TM copper depletion therapy for moderate – high risk TNBC patients
- Target patient populations include:
- TNBC non-responders to chemotherapy / immunotherapy regimens
- Treatment resistant TNBC with high risk of metastasis
- TNBC with residual disease
Technology Advantages
- TM is clinically validated agent with proven safety in placebo-controlled trials for Wilson’s disease and advanced cancer
- Oral formulation is bioavailable, pharmacodynamic biomarker (copper depletion) easily quantified via ceruloplasmin in the blood
- Ph2 showed striking 5 yr. survival benefit in stage IV pxts vs.TNBC natural history (50% EFS vs. 11%)
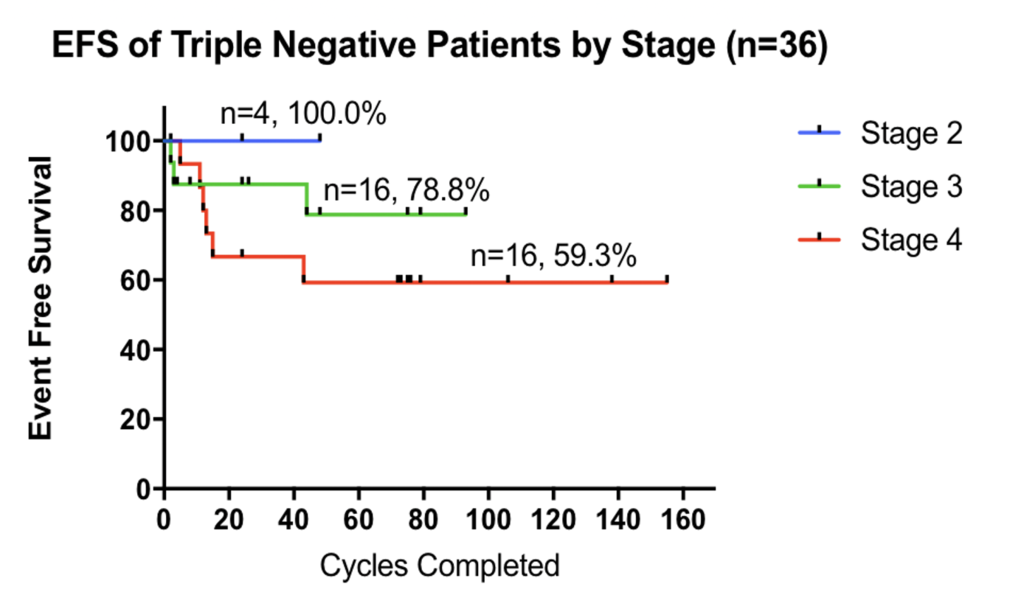
Figure 1: Phase II pilot study of TM (in addition to standard therapy) in advanced cancer patients at moderate-to-high risk of recurrence. At a median follow-up of 10.4 years, the progression-free survival for all 75 patients is 71.4% (top), including a progression-free survival rate of 83% for all stage 2/3 patients with TNBC (bottom). Cycle = 28 days.
Publications
Resources
Intellectual Property
Patents
- Provisional Application Filed
Cornell Reference
- 11001
Contact Information

For additional information please contact
Brian Kelly
Director, Business Development and Licensing
Phone: (646) 825-2766
Email: bjk44@cornell.edu

