Principal Investigator:
Nicholas Dopkins, Instructor of Biomedical Sciences in Medicine
Background & Unmet Need
- Cystic fibrosis (CF) is a genetic disorder caused by mutations in the CF transmembrane conductance regulator (CTFR) gene
- This hereditary mutation results in inadequate mucus production and compromises bacterial clearance mechanisms, predisposing CF patients to persistent lung infections
- To fight bacterial infections, cells express TLR5 in response to bacterial motor protein Flagellum (FLA), which causes an innate immune response
- Unregulated TLR5 activation in response FLA results in pathogenic inflammation
- Most notably, P. aeruginosa can cause a life-threatening infection in the pulmonary tract of CF patients by triggering excess TLR5-mediated inflammation
- Unmet Need: Anti-inflammatory therapy targeting TLR5 to provide therapeutic relief that does not drive immunotoxicity
Technology Overview
- The Technology: Repurposed reverse transcriptase inhibitors (RTis) to alleviate TLR5-driven inflammation in severe cystic fibrosis
- The Discovery: Expression of endogenous retroelements is significantly altered in the peripheral blood mononuclear cells (PBMCs) of CF patients
- Flagellum (FLA) delivery results in signaling through TLR5, affecting endogenous retroelements (EREs) downstream
- Reverse transcriptase inhibitors (RTis) selectively inhibit TLR5-induced immunity through expression of TEs
- PoC Data: Four RTis inhibited endogenous reverse transcriptase activity and the resulting TLR5-induced inflammatory response in response to FLA, including inflammatory cytokines TNFa and IL-1B
- RTis inhibited TNFa production at all concentrations tested (0.025uM-2.5 uM), and IL-1B at the highest concentration (2.5 uM)
Technology Applications
- Repurposed RTis to alleviate inflammation induced by TLR5 activation in Cystic Fibrosis
- RTis may be used as a cotreatment to alleviate TNF-driven inflammation in sepsis with S. typhi or P. aeruginosa
Technology Advantages
- RTis circumvent increasing antibiotic resistance in P. aeruginosa infection
- RTis bypass inter-individual heterogeneity and immunotoxicity
- Safety and dosages for RTis have been established for individuals on PrEP and people living with HIV
- Production of RTis in commercial quantities has been established by several manufacturers
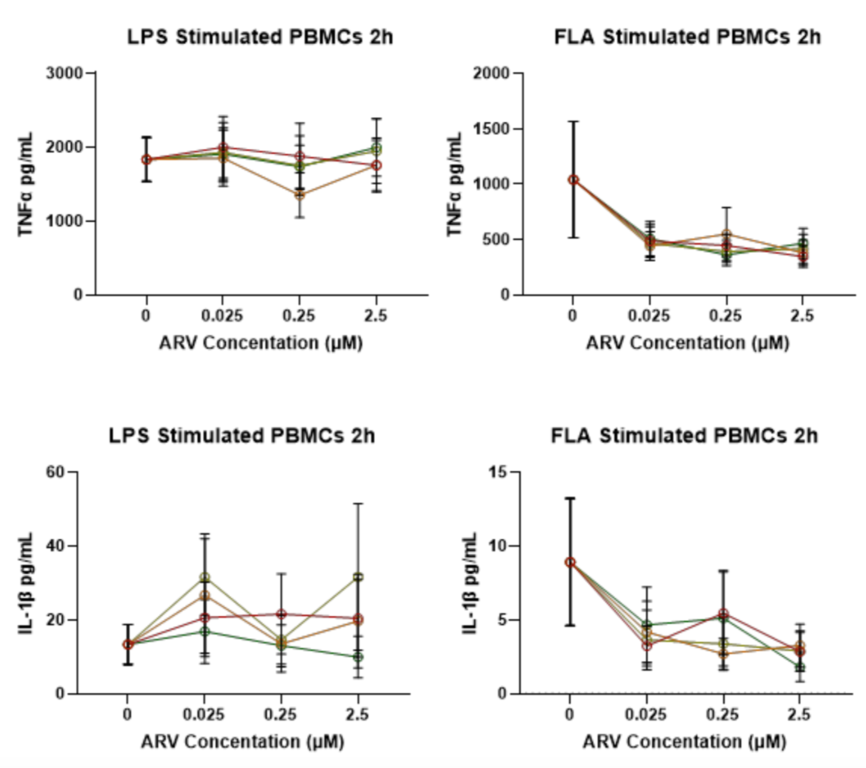
Figure 1: RTi delivery inhibits cytokine production in response to acute TLR5 activation.
Publications
Resources
Intellectual Property
Patents
- Provisional Application Filed
Cornell Reference
- 10455
Contact Information

For additional information please contact
Jamie Brisbois
Manager, Business Development and Licensing
Phone: (646) 921-4743
Email: jamie.brisbois@cornell.edu

