Principal Investigator:
Rasa Zarnegar, Professor of Surgery
Background & Unmet Need
- Gastroesophageal reflux disease (GERD), a condition in which stomach acid enters the esophagus, affects over 40 M Americans annually
- Significant discomfort and/or damage can be caused, as cells on the lining of the esophagus are not resistant to stomach acid
- Some patients do not respond to medications or become refractory and require surgical intervention
- However, current diagnostic tests to assess reflux are inadequate, as many patients do not present with classical symptoms
- It is therefore difficult to predict which patients while experience symptom resolution after surgical intervention
- Unmet Need: Improved methods of diagnosing GERD to identify patients who may benefit from surgical intervention
Technology Overview
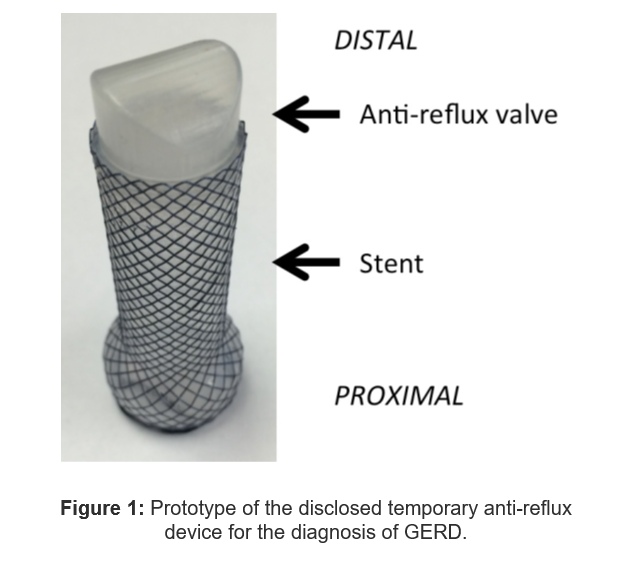
- The Technology: A temporary, endoscopically-placed device that mimics surgery by artificially recreating a normal lower esophageal sphincter
- The device spans the gastroesophageal junction, and includes an inset two-way valve that allows food to pass but prevents reflux of stomach contents
- After placement of the device, patients may be monitored for up to 30 days to assess impact on symptoms
- Successful symptom resolution increases confidence in a GERD diagnosis and indicates that a patient is likely to respond to therapeutic or surgical intervention
Technology Applications
- Temporary device for definitive diagnosis of GERD
- Identification of patients who may benefit from additional therapeutic or surgical interventions
- Semi-permanent stents for long-term management of GERD symptoms
Technology Advantages
- Temporary device that may be placed with an endoscope
- Provides a physical barrier to evaluate the need for surgical
- Pressure required to open the valve in the retrograde is sufficient to prevent reflux but still allows for belching and vomiting
Resources
Intellectual Property
Patents
- US Patent Application: US20190038393A1. "Endoscopically deployable anti-reflux device." Published Feb 07, 2019.
- EP Patent: EP3346957B1. "Endoscopically deployable anti-reflux device." Issued Mar 03, 2021.
Cornell Reference
- 6121
Contact Information

For additional information please contact
Louise Sarup
Associate Director, Business Development and Licensing
Phone: (646) 825-3932
Email: lss248@cornell.edu

