Principal Investigator:
David Lyden, Professor of Pediatrics
Background & Unmet Need
- Brain metastasis most commonly arises from lung and breast cancers, making up more than 50% of brain tumors in adults
- Despite its high lethality, brain metastasis is poorly prognosed and lacks effective therapies
- Current protocols for prognosis rely on brain scans of patients who already have symptoms, and it is difficult to predict who will develop brain metastasis
- Current treatment options include surgery, which is limited by tumor location, or radiation-based therapy, which is associated with cognitive decline. Moreover, brain metastasis has a high rate of recurrence
- Predicting brain metastases could be useful to put at-risk patients under closer surveillance and intervene before metastasis has occurred
- Unmet Need: Methods for prognosis, prevention, and treatment of brain metastasis
Technology Overview
- The Technology: A method to predict likelihood of brain metastasis based on the expression of cell migration-inducing and hyaluronan-binding protein (CEMIP) on tumor exosomes
- A therapeutic antibody targeting for prevention and treatment of cancer metastasis to the brain
- The Discovery: CEMIP is enriched in brain metastatic breast and lung tumor-derived exosomes
- Tumor-secreted factors, including exosomes, can reshape distant microenvironments, such as pre-metastatic niches, in order to drive organ-specific metastasis
- CEMIP remodels the brain microenvironment by inducing inflammation in the vascular niche, which promotes brain metastasis
- Depletion of CEMIP in tumors impairs brain metastasis, disrupting invasion and tumor cell association with the brain vasculature
- PoC Data: CEMIP was found to be enriched in the exosomes from brain metastatic cells
- Loss of CEMIP in brain-tropic breast cancer cells reduced the number of brain metastatic foci by 70%
- Patients with brain metastasis had significantly higher CEMIP expression, and metastases of the brain had higher CEMIP expression than other sites
- Patients with high levels of CEMIP expression had a shorter latency period for metastasis and significantly poorer prognoses
- Mice with brain-trophic metastatic breast cancer cells (BrT1) without CEMIP expression (KO) had 70% fewer metastatic foci and reduced metastatic burden in the brain 4 weeks post-injection compared to those with full CEMIP expression
- Antibodies to CEMIP have been generated in collaboration with the Tri-I TDI and tested for blocking capacity in vitro
Technology Applications
- Prognostic biomarker for risk of metastasis to the brain
- Method to non-invasively screen patients for primary and recurrent brain metastasis
- A biomarker for treatment selection for CEMIP-targeted therapies to prevent metastasis to the brain
- Prophylactic therapy for patients with high likelihood of metastasis to the brain
- Therapeutic treatment for patients with existing brain metastasis
Technology Advantages
- CEMIP may be detected and targetedat early stage, during pre-metastatic niche formation, which could allow for prevention of brain metastasis
- CEMIP may be detectable from patient plasma, and could serve a non-invasive biomarker for anti-CEMIP therapy
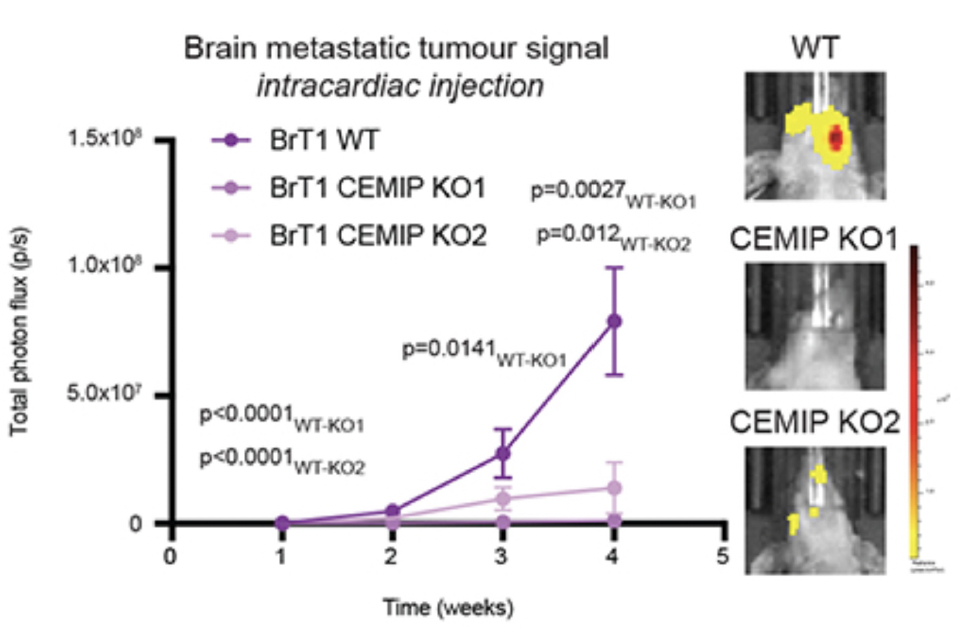
Figure 1: Mice intracardially injected with brain-trophic breast cancer metastatic cells (BrT1) with CEMIP knocked out had decreased brain metastatic tumor signal.
Resources
Intellectual Property
Patents
- US and EP Patent Applications Filed
Cornell Reference
- 7932
Contact Information

For additional information please contact
Brian Kelly
Director, Business Development and Licensing
Phone: (646) 825-2766
Email: bjk44@cornell.edu

