Principal Investigator:
Ronald G. Crystal, Professor and Chair of Genetic Medicine
Background & Unmet Need
- Eosinophils are highly specialized, bone-marrow derived, granulocytic effector cells (white blood cells) that store and release several highly active mediators
- Eosinophils are implicated in a variety of chronic allergic disorders, including asthma and eosinophilic esophagitis (EoE), as well as certain cancers (e.g., chronic eosinophilic leukemia-not otherwise specified (CEL-NOS))
- Eosinophilic disorders have largely been treated with chronic administration of corticosteroids, which are commonly linked to numerous adverse effects
- Antibody therapeutics are effective for many patients, but must be repeatedly administered for continuous efficacy
- Unmet Need: Therapeutics for eosinophilic disorders which address eosinophil accumulation and provide sustained, long-term benefits for patients
Technology Overview
- The Technology: AAV gene therapy that provides sustained in situ expression of an anti-eosinophil monoclonal antibody for eosinophilic disorders
- Various anti-eosinophil antibodies for targets such as Siglec-8 or IL-5 may be incorporated in the vector
- AAVrh.10mAnti-Eos is an rh.10 AAV vector encoding antibody Siglec F, which induces eosinophil apoptosis
- PoC Data: A single dose of AAVrh.10mAnti-Eos resulted in high, persistent serum levels of Siglec-F
- In a mouse model of CEL-NOS, a single dose of AAVrh.10mAnti-Eos provided long-term suppression of eosinophils in blood and increased survival
- In a mouse model of EoE, AAVrh.10mAnti-Eos administration reduced blood and esophageal eosinophil numbers (P < 0.02 and P < 0.002, respectively), protected from esophageal tissue remodeling, and minimized food impaction
Technology Applications
- Treatment of CEL-NOS and other leukemias where eosinophils play a prominent role
- Treatment of EoE and related eosinophilic gastric disorders
- Treatment of other disorders in which eosinophils are elevated, including allergic and endocrine disorders
Technology Advantages
- A single dose may be sufficient for sustained therapeutic effect
- Provides a platform for expression of various anti-eosinophil antibodies, such as Siglec-8 or IL-5
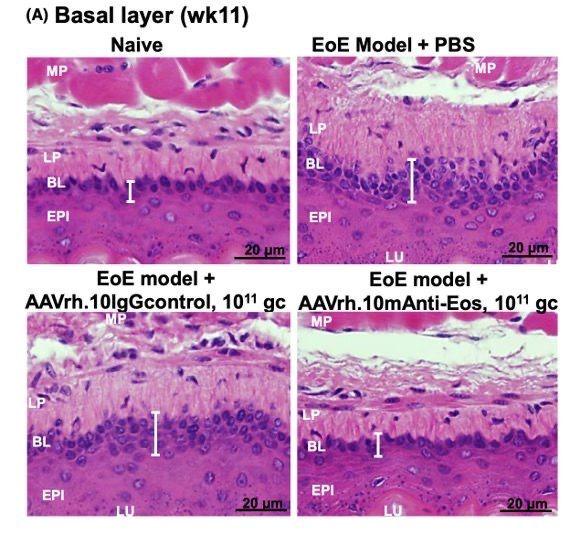
Tissue remodeling, as demonstrated by increase in area of basal lamina (white bar), was decreased in mice treated with AAVrh.10mAnti-Eos in a murine model of EoE
Publications
Resources
Intellectual Property
Patents
- US Application Filed US20200330608: "Gene therapy for eosinophilic disorders"
- EP Application Filed EP3732194: "Gene therapy for eosinophilic disorders"
- Additional Applications Filed in AU, BR, CA, CN, IL
Cornell Reference
- 7909
Contact Information

For additional information please contact
Brian Kelly
Director, Business Development and Licensing
Phone: (646) 825-2766
Email: bjk44@cornell.edu

