Tangible materials developed by Weill Cornell Medicine investigators, including antibodies, cell lines, mouse models and organoids, are valuable assets to both the research community and biomedical industry. By commercializing such materials created to conduct their own research, investigators can propel translational science as well as create revenue for their labs. Dr. Lukas Dow, a professor of biochemistry in medicine who has a decade of experience in commercializing tangible materials, shared his insights with Enterprise Innovation.

Dr. Lukas Dow (Provided)
Your lab produces genetically engineered mouse models and organoids for research, some of which have been licensed. Can you give us more background on the tools your lab has developed?
My interest is in cancer genetics – understanding the genetic changes that drive disease initiation and progression, and response to therapy. Particularly, we have been studying KRAS, a gene that encodes a protein involved in cell growth.
The KRAS gene is mutated in greater than 95% of pancreatic cancers, about 50% of colon cancers and about 25% of lung cancers. The changes almost always occur in codon 12 which normally codes for the amino acid glycine during protein synthesis. Many different mutations can occur in this one position and change the amino acid in the protein. What's curious is that some cancer types show specific changes more frequently than other cancer types.
Scientists had previously developed a mouse model for one well-known KRAS mutation called G12D. As a geneticist, I was intrigued by whether other changes in this one amino acid would produce different effects. So, we used a tool called CRISPR to precisely cut and edit the gene at specific spots, which enabled us to create mouse models more easily than previously possible.
We made a series of mice that were identical, except for the one change at the amino acid glycine in position 12. At the time, biotech and pharma companies were interested in developing drugs that targeted the KRAS protein, so these mouse models presented commercial and licensing opportunities. The first drugs to become available, both pre-clinically and clinically, specifically targeted KRAS with a G12C mutation, which changed the amino acid glycine to cysteine.
Until we developed our mouse model, testing new drugs and therapeutic combinations that inhibited the KRAS protein with the G12C mutation was difficult. As a result, we received many inquiries from pharma about licensing the G12C mouse, which would enable them to test their drugs more efficiently than in other systems.
Then, we started developing organoid models from those mice that carry many different KRAS mutations, as well as mutations in other common tumor suppressor genes like p53 or the APC (Adenomatous polyposis coli) tumor suppressor that is frequently mutated in colon cancer.
Once you have made those organoid models, they become a renewable resource. These three-dimensional cultures of cells that can be grown into mini organs and studied in cell culture dishes can be expanded and frozen. That makes them relatively easy to share. We've sent them to many academic collaborators. We also had interest from biotech and pharma about in-licensing the organoid models to test drugs quickly with different genetic models, and it’s cheaper than using the actual mouse.
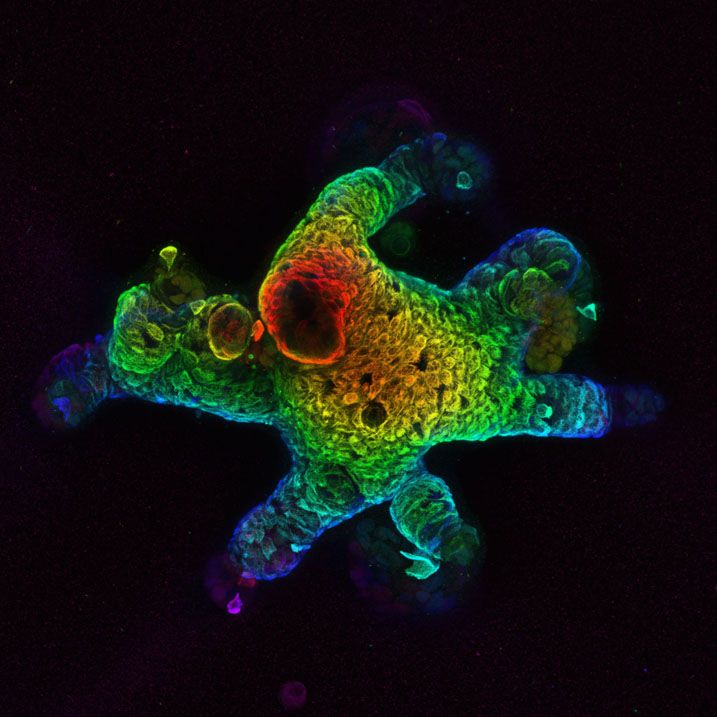
Organoid Developed by the Dow Lab (Provided)
Can you tell us more about the commercialization process?
I started doing this almost ten years ago. We presented the data at conferences and posted our manuscript as a preprint on bioRxiv so others could have free access before it was published in journals. Then, we started hearing from commercial entities asking to collaborate, to share the models and to license them. We are happy to have our models used either academically or commercially to accelerate drug development.
Although mice and organoid models can’t be patented, they're great for in-licensing because they take a lot of labor to produce and validate. Companies don't want to spend the time doing that. We worked with the Center for Technology Licensing (CTL), which helped us liaise with the different companies seeking to in-license our assets, less out of concern for patent infringement but more from a licensing standpoint.
Sometimes companies will go to Weill Cornell first. In other cases, they have reached out to me directly to have an introductory call and learn about our research before initiating licensing agreements. In some cases, companies will in-license the technology as well as create a sponsored research agreement, which is basically a grant to the lab to work on research that interests both the company and the lab. In those instances, I'm a lot more involved in the terms of the agreements. We need to clarify what the lab is providing and what the timelines are, especially in cases where the sponsor is interested in analyzing something that doesn't yet exist, but we have all of the pieces required to make it.
It took a little while to get things up and running for the first license, but after that, the process was straightforward. We're usually involved the first time an asset is licensed, and then we are pretty hands off.
Traditional funding sources don’t usually want to invest in developing tools. So, it is nice when we can build structures into the initial licensing agreements for mouse and organoid models to have some money come back into the lab to cover the developmental costs.
It's also fantastic to supplement income for the inventors in the lab. Inventors who created the systems can get royalties and feel good about people using those tools.
What have you learned as a scientist about managing these kinds of non-traditional assets?
For the mice, it was super easy—we used a third-party vendor to distribute them. We distribute our models through The Jackson Laboratory because that's the worldwide go-to place for mouse models. The Jackson Lab offers the added benefit of cryopreserving the models in case we need to get them back at some point. It serves both as the supplier academically and acts as a contract research organization for distributing mice to commercial entities. We've also used Charles River Labs for moving mice to companies.
There have been some challenges around developing license agreements for things that don't already exist. Those timelines can be difficult to predict. Sometimes it happens faster than you expect, but more often, things are a bit slower. It could be stressful for both sides when there are delays because things aren’t working or if a contamination during the production process means starting over.
In some cases, I've been involved in mediating the discussions with CTL and the lawyers on the company side. I try to ensure that our license agreements are set up so that we aren’t over or underdelivering—being a bit more conservative about what we bake into agreements.
What advice do you give fellow investigators who have created non-traditional assets with commercial potential?
I think being open and aware of potential opportunities is half the battle.
Be open to options because it's not a lot of work from the investigator’s side for a potential upside financially. Be selective about which opportunities are real and serious when companies reach out for informational meetings. Use that 15-minute chat to let people know what you have. Talk to people at conferences as well – you often get approached for in-licensing.
Be upfront and honest about what you have, but you need to be careful about not disclosing too much. For instance, don't show slides with structures of molecules. The institution can provide investigators who are interested in commercialization training to prepare them for these kinds of discussions. It's important that everybody understands what can and can't be disclosed when you have new technologies and new inventions.
Most companies are fine if you speak in broad terms. Generally, it's important to understand what they're looking for and to be objective. Don’t oversell. From a transactional perspective, companies are willing to pay for what's tangible and exists, and they want to know what you can accomplish or what's possible.
Since CTL exists, it doesn’t take a huge effort once you pass those first introductory meetings. I believe sharing your technologies and letting people use what you've built is fulfilling in the long term.
Many Weill Cornell Medicine physicians and scientists maintain relationships and collaborate with external organizations to foster scientific innovation and provide expert guidance. The institution makes these disclosures public to ensure transparency. For this information, see profile for Dr. Lukas Dow.

