Stephen Kaminsky, Professor of Research in Genetic Medicine
Summary
- Fuchs endothelial corneal dystrophy (FECD) is the most common reason for cornea transplantation, and is characterized by progressive loss of corneal endothelial cells and the formation of deposits calledguttae
- This loss of corneal endothelial cellseventually leads to corneal edema and progressive vision loss
- The cells in the corneal endothelium monolayer, which is critical for transporting fluid out of the cornea, are terminally differentiated and cannot be regenerated
- When the density of corneal endothelium drops below 500 cells/mm2, the cornea swells, leading to vision loss
- There is extensive evidence that the primary cause of FECD is exposure to oxidants from ultraviolet (UV) light that progressively damage the corneal endothelium
- Dr. Kaminsky has hypothesized that the corneal endothelium can be protected from oxidant stress by using in vivo gene therapy in the eye anterior chamber to provide a persistent anti-oxidant shield with minimal inflammation
Technical Overview
- The Kaminsky lab plans to leverage AAV-mediated delivery of two major antioxidant enzymes to provide persistent protection against environmental stimulants of oxidant stress to the corneal endothelium
- The target for anti-oxidant protection is the endothelial layer of the cornea, the site of Fuchs pathology
- The primary goal for the therapy will be to protect the corneal endothelium from UV-mediated oxidant stress without causing inflammation within the anterior chamber, the corneal surface, or the vitreous
- The lab intends to identify a gene therapy that will generate a persistent antioxidant shield sufficient to protect the corneal endothelium from oxidant stress and minimize any inflammatory response to the vector
Market Opportunity
- FECD is estimated to affect ~300 million adults over 30, and is expected to rise to 415 million by 2050
- The only current treatment for FECD is corneal transplant, and an estimated 15-20% of patients require this treatment
- However, corneal transplant has a high rate of failure, representing a high unmet need for patients
Partnering Opportunity
Weill Cornell Medicine is seeking an industrial partner with deep domain expertise and a strong presence in the gene therapy or ophthalmology space to advance the development of this therapy for FECD
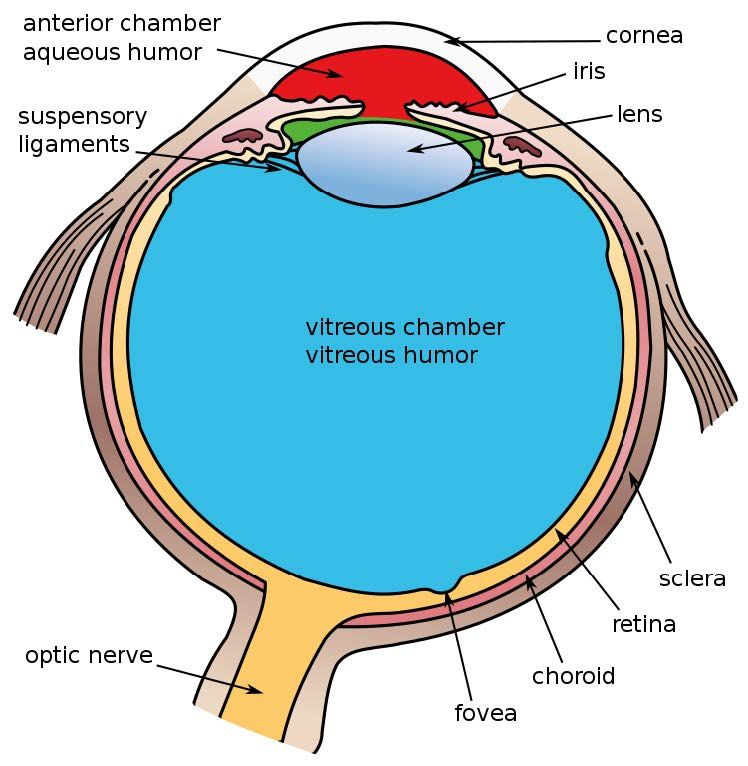
Figure 1: Schematic of the anatomy of the eye *Vipin et al. Indian J Ophthalmol. 2022.
Contact Information

For additional information please contact
Brian Kelly
Director, Business Development and Licensing
Phone: (646) 825-2766
Email: bjk44@cornell.edu

