Ronald G. Crystal, Professor and Chair of Genetic Medicine
Background & Unmet Need
- Alpha 1-antitrypsin (AAT) deficiency is a hereditary disorders characterized by low serum levels of functional AAT, and is associated with early development of panacinar emphysema
- AAT deficiency affects ~90 K patients in the U.S., and is caused by mutation in the SERPINA1 gene
- AAT inhibits serine proteases, including neutrophil elastase, protecting the lung from proteolytic destruction
- The current SOC for AAT deficiency is weekly infusions of purified AAT from pooled human plasma
- While AAT augmentation therapy is safe and reduces the rate of lung destruction, it requires burdensome weekly parenteral infusions and carries risks of allergic reactions and viral contamination of human plasma-derived products
- Unmet Need: Therapeutic strategy to augment AAT levels in a safe and convenient format
Technology Overview
- The Technology: AAV8 gene transfer vector coding for an oxidant-resistant form of AAT for the treatment of AAT deficiency
- The oxidation-resistant AAT (A213/V351/L358) maintains antiprotease activity under oxidant stress compared to wild-type and other engineered variants
- PoC Data: A single dose of 8/AVL afforded sustained serum AAT levels, in both male and female mice, with similar absolute AAT levels for both IV and IPL delivery
- However, IPL delivery provided a significantly higher lung epithelial lining fluid (ELF) to serum ratio of AAT than IV delivery, suggesting IPL is the preferred delivery route
- Mice administered the 8/AVL vector retain anti-NE activity in the ELF 24 weeks after treatment, whereas mice receiving the WT 8/AMM vector display no anti-NE activity under oxidizing conditions
Technology Applications
- One-time treatment of AAT deficiency
- Provides a general strategy for optimizing gene therapy for other diseases associated with enzymatic deficiency
Technology Advantages
- The modified AAT protein is highly resistant to oxidant stress and less susceptible to potential anti-AAT immunity than the wild-type protein
- Single dose treatment significantly improves patient quality of life compared to weekly infusions
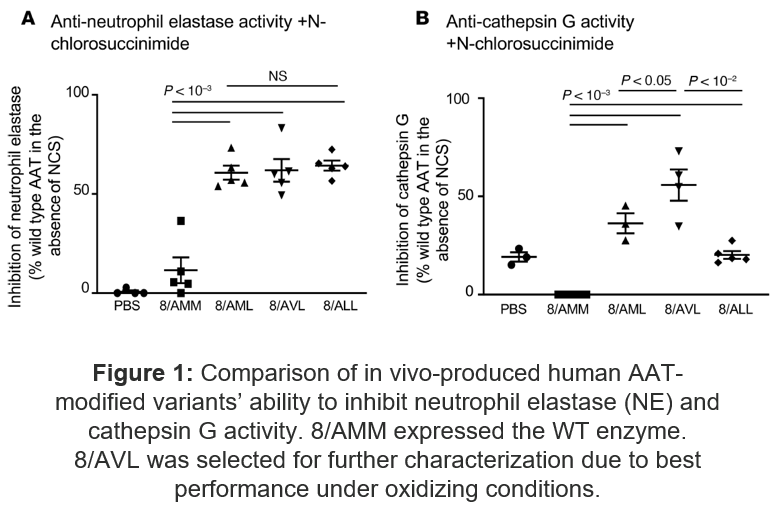
Figure 1: Comparison of in vivo-produced human AATmodified variants’ ability to inhibit neutrophil elastase (NE) and cathepsin G activity. 8/AMM expressed the WT enzyme. 8/AVL was selected for further characterization due to best performance under oxidizing conditions.
Resources
Intellectual Property
Patents
- US Patent Application: US20200102575A1. "Oxidation-resistant aat gene therapy." Published Apr 2, 2020.
- EP Patent Application: EP3600345A4. "Oxidation-resistant aat gene therapy." Published Jan 6, 2021.
Cornell Reference
- 7690
Contact Information

For additional information please contact
Brian Kelly
Director, Business Development and Licensing
Phone: (646) 825-2766
Email: bjk44@cornell.edu

